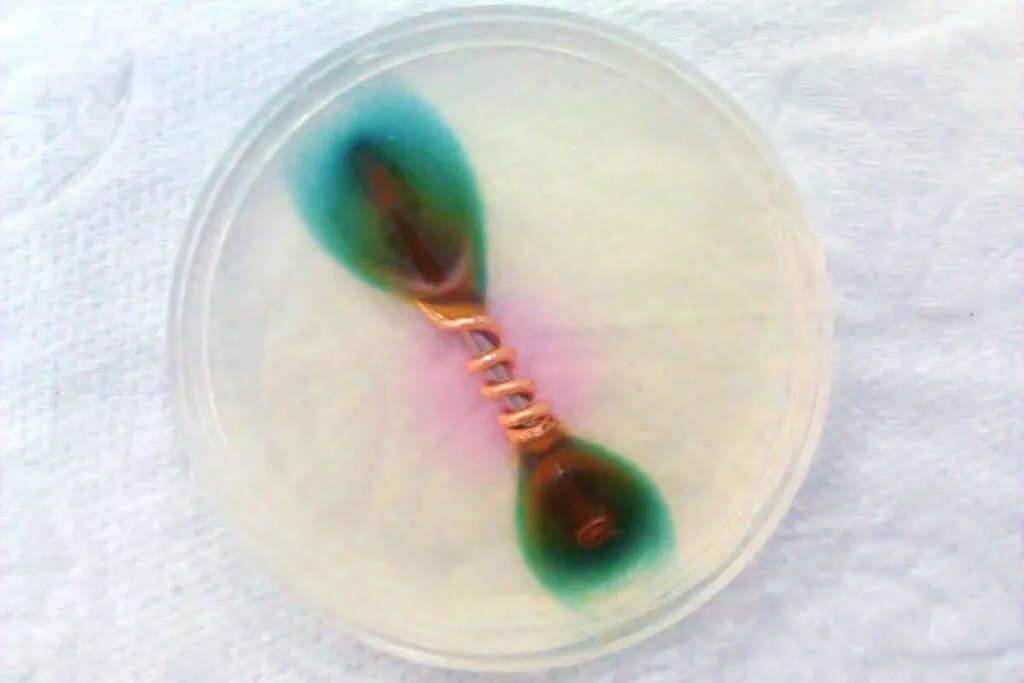
Definition of Galvanic Corrosion
Galvanic corrosion occurs when two different metals connect in an electrolyte, such as saltwater or moisture. In this electrochemical reaction, one metal becomes the anode and corrodes more rapidly, while the other becomes the cathode and remains protected. You often see it when steel screws join aluminum or brass components. Over time, this process can weaken connections and lead to leaks or part failure. The severity depends on the metal pair, surface finish, and environmental factors like temperature and chloride levels.
Engineers and buyers must consider material compatibility, protective coatings, or insulating barriers to prevent damage. Understanding galvanic corrosion helps you select durable fastener materials and ensure long-lasting assemblies.
Why Galvanic Corrosion Matters for Screws and Fasteners
When you mix metals in an assembly, galvanic corrosion can attack the weaker metal and undermine joint integrity. This leads to unexpected maintenance, part replacement, and potential safety issues. By recognizing galvanic risks early, you can choose compatible metals or add barriers to extend service life.
In business terms, preventing galvanic corrosion reduces warranty claims and downtime. You maintain customer trust by delivering reliable products. Proper material selection and protective measures also lower life-cycle costs for your projects.
Related Terms
Electrochemical SeriesCathodic Protection
Anodic Metal
Galvanic Series
Corrosion Inhibitor
Passivation
FAQ
What causes galvanic corrosion in screws?
It occurs when a screw made of one metal touches a component of another metal in the presence of an electrolyte. Differences in electrochemical potential drive ion movement, corroding the more active (anodic) metal.
How can you prevent galvanic corrosion in fasteners?
Use the same metal for mating parts or choose metals close in the galvanic series. Apply protective coatings, use insulating washers or sleeves, and seal joints to keep moisture and salts away.
Which metal pairs are most susceptible to galvanic corrosion?
High-risk pairs include steel-aluminum, copper-steel, and brass-aluminum. The greater the electrochemical potential difference, the faster the anodic metal will corrode.